Scientists at Cedars-Sinai have uncovered an unexpected way the nervous system repairs itself after injury, one that involves support cells acting far from the original damage. Their findings reveal how specialized astrocytes communicate with immune cells to clear debris and reduce inflammation, a process that appears crucial for recovery. Credit: Stock
A Cedars-Sinai study has identified a previously unknown role for astrocyte cells in how the brain responds to damage and disease.
Cedars-Sinai researchers have identified a biological repair process that may eventually contribute to new treatments for spinal cord injuries, stroke, and neurological diseases such as multiple sclerosis. The research, published in Nature, reveals an unexpected role for astrocytes, cells that support and regulate activity in the central nervous system.
“Astrocytes are critical responders to disease and disorders of the central nervous system—the brain and spinal cord,” said neuroscientist Joshua Burda, PhD, assistant professor of Biomedical Sciences and Neurology at Cedars-Sinai and senior author of the study. “We discovered that astrocytes far from the site of an injury actually help drive spinal cord repair. Our research also uncovered a mechanism used by these unique astrocytes to signal the immune system to clean up debris resulting from the injury, which is a critical step in the tissue-healing process.”
The research team named this newly identified group of cells “lesion-remote astrocytes,” or LRAs, and found that they exist in multiple distinct forms. For the first time, the study explains how one specific LRA subtype can detect damage from a distance and activate a response to injury.
Understanding Astrocytes and the Spinal Cord
The spinal cord is a long structure made of nerve tissue that extends from the brain down through the spine. Its inner region, known as gray matter, contains nerve cell bodies along with supportive cells such as astrocytes.
Encasing the gray matter is white matter, which consists of astrocytes and elongated nerve fibers that carry signals between the brain and the rest of the body. By maintaining the environment around these nerve fibers, astrocytes play a vital role in keeping communication within the nervous system functioning properly.
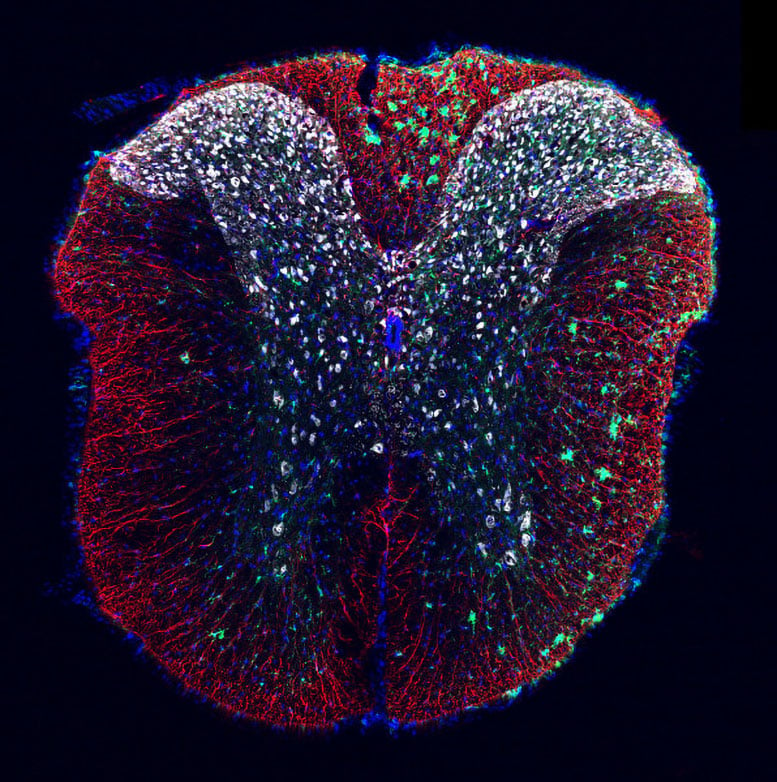
This mouse spinal cord tissue cross section shows lesion-remote astrocytes (LRAs) in red alongside clusters of debris-clearing microglia in green. Credit: Sarah McCallum, PhD, of the Burda Lab at Cedars-Sinai.
Spinal cord injuries damage nerve fibers, paralyzing parts of the body and disrupting sensory input such as touch and temperature. The severed fibers die off and become debris. In most other types of tissue in the body, inflammation takes place only at the site of injury. But because of the length of nerve fibers in the spinal cord, damage and inflammation extend far beyond the injury site.
Lesion-Remote Astrocytes and Immune Signaling
Investigators looked at laboratory mice with spinal cord injury and found that LRAs play an important role in supporting nervous system repair. They saw strong evidence of the same mechanism in tissue samples from human patients with spinal cord injury.
The Burda Lab identified one LRA subtype that sends out a protein called CCN1 to signal to immune cells called microglia.
“One function of microglia is to serve as chief garbage collectors in the central nervous system,” Burda said. “After tissue damage, they eat up pieces of nerve fiber debris—which are very fatty and can cause them to get a kind of indigestion. Our experiments showed that astrocyte CCN1 signals the microglia to change their metabolism so they can better digest all that fat.”
Burda said this efficient debris clearing might have a role in the spontaneous recovery found in many patients with spinal cord injury. In the absence of the astrocyte-derived CCN1 protein, the investigators found that recovery is drastically impaired.
“If we remove astrocyte CCN1, the microglia eat, but they don’t digest. They call in more microglia, which also eat but don’t digest,” Burda said. “Big clusters of debris-filled microglia form, heightening inflammation up and down the spinal cord. And when that happens, the tissue doesn’t repair as well.”
Broader Implications for Neurological Disease
When investigators looked at spinal cord tissue from human patients with multiple sclerosis, they found the same mechanism at work, Burda said. He added that these fundamental principles of tissue repair likely apply to any sort of injury of the brain or spinal cord.
“The role of astrocytes in central nervous system healing is remarkably understudied,” said David Underhill, PhD, chair of the Department of Biomedical Sciences. “This work strongly suggests that lesion-remote astrocytes offer a viable path for limiting chronic inflammation, enhancing functionally meaningful regeneration, and promoting neurological recovery after brain and spinal cord injury and in disease.”
Burda is now leading efforts to harness this CCN1 mechanism in spinal cord healing and to further investigate the role of astrocyte CCN1 in inflammatory neurodegenerative disease and in aging.
Reference: “Lesion-remote astrocytes govern microglia-mediated white matter repair” by Sarah McCallum, Keshav B. Suresh, Timothy S. Islam, Manish K. Tripathi, Ann W.
Saustad, Oksana Shelest, Aditya Patil, David Lee, Brandon Kwon, Katherine Leitholf, Inga Yenokian, Sophia E. Shaka, Connor H. Beveridge, Palak Manchandra, Caitlin E. Randolph, Gordon P.
Meares, Ranjan Dutta, Jasmine Plummer, Vinicius F. Calsavara, Riki Kawaguchi, Simon R. Knott, Gaurav Chopra and Joshua E. Burda, 17 December 2025, Nature.
DOI: 10.1038/s41586-025-09887-y
Funding: This work was supported by: the US National Institutes of Health (NIH) 5R01NS128094, R00NS105915, K99NS105915 (to J.E.B.), F31NS129372 (to K.S.), K99AG084864 (S.M.) R35 NS097303 and R01 NS123532 (RD), R01MH128866, U18TR004146, P30 CA023168 and ASPIRE Challenge and Reduction-to-Practice award (to G.C.); the Paralyzed Veterans Research Foundation of America (to J.E.B.); Wings for Life (to J.E.B.); Cedars-Sinai Center for Neuroscience and Medicine Postdoctoral Fellowship (to S.M.); American Academy of Neurology Neuroscience Research Fellowship (to S.M.); California Institute for Regenerative Medicine Postdoctoral Scholarship (to S.M.); The United States Department of Defense USAMRAA award W81XWH2010665 through the Peer Reviewed Alzheimer’s Research Program (to G.C.); The Arnold O. Beckman Postdoctoral Fellowship (to C.E.R.); The Purdue University Center for Cancer Research funded by NIH grant P30 CA023168 is also acknowledged.
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.





Comments(0)
Join the conversation and share your perspective.
Sign In to Comment